CH3121 Chemical, Biological & Plant Safety Individual Continuous Assessment 2, 2025, NTU Singapore
| University | Nanyang Technological University (NTU) |
| Subject | CH3121 Chemical Biological & Plant Safety |
Quiz: Source, Dispersion and LOPA Analysis- Individual CA2
- Perform Analysis using Source Model
- Deploy ALOHA software and generate the toxic and dispersion contours.
- Summarize the results and do a comparison through your observation.
- Perform LOPA to develop extra independent protection layer(s)
Problem:
An ammonia storage tank leaks at its bottom piping through an effective opening with a diameter of 50 mm. NH3 is stored under saturated liquid conditions at 11.58 bar and 30oC. Location of leak in piping is at 10-meter elevation above the ground level. Typical details of the NH3 storage tank are shown in the following diagram.
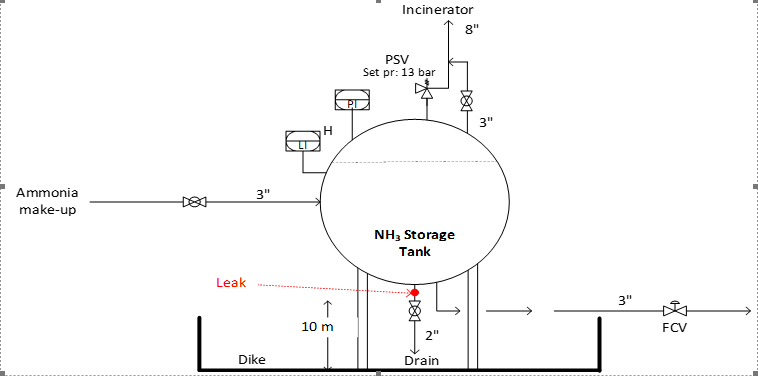
a. Estimate NH3 liquid spill (in kg) collected in the bottom dike in one hour. (Assume (i) steady state between spill rate and evaporation rate. (ii) Evaporation occurs over entire cross section of the dike). Estimate the total NH3 vapor release to air by flashing and evaporation.
NH3 liquid temperature after depressurizing through 50 mm opening to atmospheric pressure (atmospheric boiling point of NH3) = – 33oC
Ammonia liquid density at -33oC = 673.1 kg/m3
Heat of vaporization of NH3 at atmospheric pressure= 1170.87kJ/kg
Difference in specific volume between vapor and liquid NH3= 1.38115 m3/kg
Cp for NH3= 2.103 kJ/kg.K
Dike dimensions: 10m(L) x 10m(B) x 0.6 m(H). (8 Marks)
b. Perform toxic dispersion analysis for released NH3 using ALOHA software. Assume open country conditions and atmospheric stability class B. Wind speed is 2 m/s. Assume a humidity of 80%. Site’s ambient temperature is 30oC. Use ALOHA Gaussian and Heavy gas dispersion models to develop ERPG1, ERPG2 and IDLH risk contours. State any assumptions used in the model. (6 Marks)
c) Perform LOPA and develop an appropriate solution to reduce the NH3 storage tank overpressure frequency to well within the acceptable frequency. Due to changes in NH3 make-up rate (much higher than the original design rate), PSV on top of the NH3 storage tank is under sized. Operator mistake during NH3 make-up can result in overpressure in the tank. State any assumptions used. Use typical PFD (probability of failure on demand) values of protection layers from lecture notes/ textbook or any other reference (If external source of reference is used, please cite it clearly). If SIS (safety instrumented system) solutions are required, develop an SRS (Safety requirement specifications) document. (6 Marks)
Initiating event frequencies for overpressure are:
| Initiating event | Frequency |
| Operator failure | 10-1 per year |
Acceptable overpressure frequency for the site is 10-5 incidents/year.
– submit via “Assignments” portal on Blackboard by 2359 hrs. 7th Nov 202
Hire a Professional Essay & Assignment Writer for completing your Academic Assessments
Struggling with your CH3121 Chemical, Biological & Plant Safety CA2 at NTU Singapore? Don’t stress over ALOHA dispersion analysis or complex LOPA calculations. Our My Assignment Help SG provide step-by-step, human-written NTU assignment help for ammonia leak modelling, vapor dispersion, and safety layer design. Get Expert Assignment Help Singapore now and submit accurate, high-quality work before the NTU deadline.
- SC1007 Data Structures & Algorithms Assignment Question 2026 | NTU
- SOC309 Contemporary Social Theory Assignment Question 2026 | SUSS
- MKTG2060 International Marketing Assessment 1 Guidelines 2026 | UON
- GPS2301 Interventions & Strategies in Special Needs Education Assignment 2026 | TP
- PSB7022CL Marketing in a Global Age Assignment 2, 2026 | Coventry University
- PSB5045EE Analog and Digital Electronics (ADE) Assignment Questions 2026
- LG71011 Cold Chain Logistics Assessment Project 2026 | ITE College
- BUSM2578 Integrated Perspective on Business Problems Assignment 1 2026 | RMIT
- GSS502 Global Security, Strategy and Leadership Tutor-Marked Assignment – 01, 2026 | SUSS
- MGT567 Strategic Human Capital and Talent Management End-of-Course Assessment 2026 | SUSS


