CH2123 Chemical Thermodynamics Assignment Questions 2026 | NTU
| University | Nanyang Technological University (NTU) |
| Subject | Chemical Thermodynamics |
CH2123 Assignment Questions
Instructions:
- This is an individual assignment. Any form of plagiarism will be penalized according to NTU’s academic integrity policy.
- Answer all 3 (three) questions. If necessary, you may need to find additional information from external resources and provide the reference you use.
- Submit your answers to Turnitin on NTULearn by 21 April 2025 at 17:00. Please only do a single file submission. Late submission will not be accepted; therefore please submit early to avoid any technical issue.
- The solutions can be handwritten (make sure it is legible) or typed. You can submit the soft copy, scanned or pictures of the solutions. Please ensure high resolution of the picture before submission.
- Please combine all of your workings, including Excel file, into one ZIP file and submit it to Turnitin.
- Homework will be graded on efforts, not the final answer obtained. However, give your best effort in solving the problems to enforce the concepts you have learned. Below is the detailed rubric for the continuous assessment, which the homework is a part of.
Question 1 (Fugacity of real gas)
By using Lee/Kesler generalized correlation below, calculate the fugacity of isobutane at 154 °C and 8620 kPa.
Generalized correlation defines the compressibility factor of a pure component as:
Z = Z₀ + ωZ₁
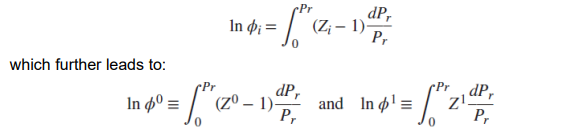
where Z₀ and Z₁ are functions of (Pr, Tr), which are the reduced pressure and reduced temperature, respectively, and ω is the accentricity factor of the pure component.
The equation for fugacity coefficient φi can now be written in terms of reduced pressure, which further leads to:
(Refer to Appendix D Tables D.13-D16, Smith Van Ness, Introduction to Chemical Engineering Thermodynamics 8th Ed., 2018)
Question 2 (Vapor-Liquid equilibrium model)
The vapor-liquid equilibrium data for a binary liquid solution containing acetone (1) – methanol (2) at 55 °C is given in Table 1. Assume that the vapor phase can be considered as ideal gas mixture whereas the activity coefficients for acetone (1) and methanol (2) can be expressed by Van Laar model as follows:
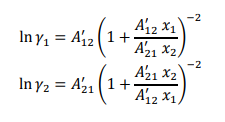
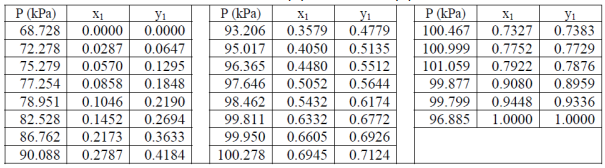
- Determine the parameters A’₁₂ and A’₂₁ (both of which are constants) that best fit the data in Table 1.
- By using Excel, construct P-x, y diagram at 55 °C based on the suggested model above, and compare it with the experimental data (see Table 1).
- Compare the azeotropic composition based on the experiment and the model above.
Note: The saturated vapor pressures of each component can be determined by using Antoine equations from any textbook/other reference.
Table 1. VLE data for acetone (1) – methanol (2) mixture at 55 °C.
Question 3 (Henry’s law application)
Aqueous solution of perfluorotributylamine can be considered as artificial blood because of its high oxygen solubility.
At 25 °C and an oxygen pressure of 1 atm, 380 mL of oxygen gas (measured at 25 °C and 1 atm) dissolves in 1 liter of pure perfluorotributylamine, which has a liquid density of 1.88 g/mL.
- Determine the Henry’s Law constant, in unit of atm, for oxygen dissolved in pure perfluorotributylamine.
- Suppose a solution contains 10 vol% perfluorotributylamine and 90 vol% water. Estimate the volume of oxygen gas (measured at 25 °C and 1 atm) dissolved in a liter of the solution when it is equilibrated with air at 25 °C. Note that the Henry’s constant for oxygen in water at 25 °C is 4.34 × 10⁴ atm.
Note: The physical properties of perfluorotributylamine can be obtained from external reference.
Struggling with CH2123 Chemical Thermodynamics Assignment? Get Expert Help Today
Many NTU students find CH2123 challenging because topics like fugacity calculations, VLE modelling, Lee–Kesler correlation, and Henry’s Law applications require strong conceptual clarity and accurate numerical work. That’s why students turn to My Assignment Help, where subject experts provide chemical thermodynamics assignment help aligned with NTU marking criteria. We deliver 100% human-written, plagiarism-free solutions with detailed step-by-step workings and Excel support. You can confidently use our NTU assignment writing service for a customised solution. For assurance, explore our engineering assignment sample.
- S2440C Health Psychology Assignment Brief 2026 | Republic Polytechnic
- HS2255 Law & Ethnics Assignment Brief 2026 | Nanyang Polytechnic
- OMGT2229 Strategic Supply Chain Assignment Question 2026 | RMIT University
- CP3404 Information Security Assignment Brief 2026 | James Cook University
- BME317 Biomedical Devices Innovation Tutor-Marked Assignment Questions 2026 | SUSS
- MKT362 Pricing End-of-Course Assessment Question 2026 | SUSS
- PSY 101 Introduction to Cultural Psychology Assessment 2026 | MU Singapore
- MKT371 Customer Insights and Analytics End-of-Course Assessment Question 2026 | SUSS
- MN3496K Clusters, Small Business and International Entrepreneurship Assignment 2026 |
- BM4708 Managing Hybrid Events Individual Assignment 3 2026 | Nanyang Polytechnic


