A2349C Pharmaceutical Legislation Graded Assignment: Regulatory Approval Plan for Glucura
| University | Republic Polytechnic (RP) |
| Subject | Pharmaceutical Legislation |
INSTRUCTIONS TO CANDIDATES
1. This individual graded assignment will constitute 100% of the overall module marks.
2. Graded assignment is to be submitted via SA 2.0 by 20 February 2025, 23 59 hrs.
3. Late submission of graded assignment without leave of absence (LOA) for the module will be subjected to the following late penalty:

Note: Late submission penalty will apply only to the component(s) that relate to the submitted work. Time after submission deadline includes weekends & Public Holidays.
4. Graded assignment should be uploaded into SA2.0 before the stipulated deadline. Save your graded assignment in word document format, as “_.doc”.
5. Complete the Graded Assignment Declaration on Page 2 and upload into SA2.0 along with your assignment as one file.
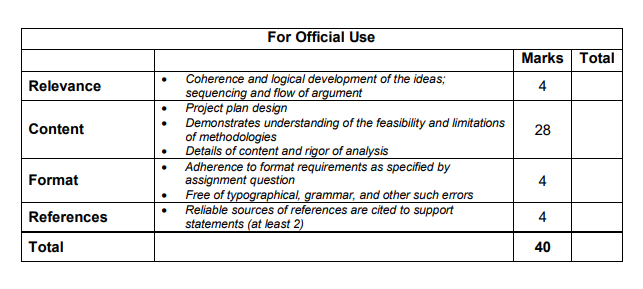


Case Study
Singapore-based company, Cura Pharmaceuticals, has local research,
manufacturing and warehousing facilities. They recently completed clinical trials for their new anti-diabetic drug, the first in its pharmacological class, and have decided to name the product Glucura.
As the Regulatory Affairs Manager at Cura Pharmaceuticals, your task is to navigate the Health Sciences Authority (HSA)’s licensing processes to obtain approval for the manufacture, distribution and sale of Glucura in Singapore. You are responsible for ensuring that Glucura complies with all the regulatory requirements and to successfully obtain all the required registration and licences for this product.
You are to prepare a set of no more than 10 presentation slides for the purpose of presenting your plan to obtain the necessary approvals for Glucura to the management of Cura Pharmaceuticals.
Some guidelines to help you prepare your plan are as follows:
Identify the type of product that Glucura is, in legislative terms.
Identify which legislation or guidelines that Glucura is regulated under.
Identify the required registration and/or licensing for Glucura, taking into account
all the relevant requirements, guidelines and/or exemptions for each of the
procedures.
Include any other factors that Cura Pharmaceuticals need to consider while
obtaining the required registration and licences for Glucura.
Justify your points wherever necessary.
Include at least 2 references for your presentation.
- GSS502 Global Security, Strategy and Leadership Tutor-Marked Assignment – 01, 2026 | SUSS
- MGT567 Strategic Human Capital and Talent Management End-of-Course Assessment 2026 | SUSS
- PSB7006CL Entrepreneurship Business Plan Assessment Coursework 1 2026 | PSB
- FMT321 Fire Safety & Engineering Tutor-Marked Assignment – 02, 2026 | SUSS
- B3029C Career Counselling Project Coursework Assessment 2026 | RP
- MTD115 Fundamentals of Digital Photography Tutor-Marked Assignment Jan 2026 | SUSS
- HS6433 Health Promotion and Counselling Assignment 2026 | NYP Singapore
- BCEC002 Business Economics Individual Assignment Brief 2026 | TP Singapore
- PSBA300CA / PSBA300CW Academic Writing 3 Assignment 2026 | PSB Academy
- PSS304 Psychological Perspective to Public Safety Assignment Questions 2026 | SUSS


